ORIGINAL RESEARCH | https://doi.org/10.5005/jp-journals-10015-1762 |
Comparing Quality and Quantity of Dentin Bridge Formed Using Mineral Trioxide Aggregate, Biodentine, and Propolis: A Double-blinded Randomized Controlled Clinical Trial
1,2Department of Conservative Dentistry and Endodontics, Saveetha Dental College, Saveetha Institute of Medical and Technical Sciences, Chennai, Tamil Nadu, India
Corresponding Author: Sindhu Ramesh, Department of Conservative Dentistry and Endodontics, Saveetha Dental College, Saveetha Institute of Medical and Technical Sciences, Chennai, Tamil Nadu, India, Phone: +91 9840136543, e-mail: drsinsushil@gmail.com
How to cite this article Mohanty S, Ramesh S. Comparing Quality and Quantity of Dentin Bridge Formed Using Mineral Trioxide Aggregate, Biodentine, and Propolis: A Double-blinded Randomized Controlled Clinical Trial. World J Dent 2020;11(5):373–379.
Source of support: Nil
Conflict of interest: None
ABSTRACT
Aim and objective: To compare the quality or quantity of dentin bridge formation of Biodentine and Propolis with the current gold standard—mineral trioxide aggregate (MTA) when used as a direct pulp-capping agent.
Materials and methods: Randomized clinical trial on 102 teeth (patients aged 13–30 years) selected for extraction due to orthodontic treatment was carried out by a single operator using group I—MTA, group II—Biodentine, and group III—Propolis as vital pulp therapy agents. The patients and the outcome assessor were blinded. After 3 months of follow-up, the teeth were extracted and sent for the histopathological analysis. The Chi-square test was applied on the data for statistical analysis.
Results: Pulp sensibility was maintained for all assessed teeth after the follow-up period. The difference between group I and group II was statistically insignificant. Group III showed significant differences in terms of morphology, continuity, and thickness when compared to the other two groups (p = 0.0).
Conclusion: The three groups successfully aided in forming dentin bridges in all the assessed teeth after follow-up. The quality and quantity of dentin bridge formation of group I—MTA and group II—Biodentine were superior than group III—Propolis. Owing to its anti-inflammatory, antimicrobial, and tissue regeneration properties, Propolis improves wound healing and can be successfully used as an agent in direct pulp capping.
Clinical significance: Biodentine is an effective pulp capping agent and is comparable to MTA. Among other advantageous properties, Propolis is an organic anti-inflammatory material that can be successfully used as a direct pulp-capping material due to its ability to induce reparative dentin, which is good enough to maintain pulp sensibility over a long period of time.
Keywords: Biodentine, Mineral trioxide aggregate, Propolis, Pulp capping..
INTRODUCTION
Direct pulp capping is a vital pulp therapy procedure in which a biocompatible material is placed over the exposed pulp and the area is sealed with a permanent filling. Any stimuli on the pulp promotes reparative dentin formation that acts as a natural barrier against it.1,2 Direct pulp capping catalyzes reparative dentin formation over the site of pulpal exposure.
With superior and more hemogeneous dentin bridge forming ability, mineral trioxide aggregate (MTA) gained steady popularity over the decade.3–11 But, over the years, researchers discovered its disadvantages of difficult handling and application, a longer binding duration, and a relatively high cost.12–14
Biodentine is a more recent calcium silicate-based dentin substitute that overcomes the various disadvantages of MTA, mostly due to minor modifications in its composition.4,15 In several systematic reviews, Biodentine has exhibited similar clinical results as MTA minus the shortcomings of the latter, proving its clinical potential time and again.16,17
Much like any other field, dentistry is also going organic gradually. Steady and successful research has been carried out observing the in vitro success of many herbal products including Zingiber officinalis,18 aloe vera gel,19 hexane extract of Mimusops elengi seeds,20 eucalyptus globules,21 and apple cider inegar.22 Propolis is a resinous material collected by honey bees from shrubs and trees. It is known to be a cariostatic agent, tested as an intracanal medicament23,24 and “as a storage medium for avulsed teeth.”25,26 Many in vitro studies and animal studies have shown that the ethanolic extract of Propolis showed bone-regenerating properties and hard tissue formation in pulpotomy.27,28 But due to the lack of any long-term randomized clinical trials to date, its histological as well as clinical potential has not been clearly justified. Thus, the aim of the present study is to compare the quality and quantity of dentin bridge formation of Biodentine and Propolis with current gold standard—MTA used as a direct pulp-capping material. The objectives of this long-term randomized clinical trial were:
- To evaluate the pulp sensibility of the treated tooth.
- To evaluate and compare, histologically, the dentin bridge continuity, morphology, and thickness using a standard grading system.
MATERIALS AND METHODS
This is a prospective, single-center, double-blinded, randomized controlled clinical trial.
Ethical Approval
The study was conducted at the Department of Conservative Dentistry and Endodontics, Saveetha Dental College, SIMATS, Chennai, India. The study protocol was approved by the institutional ethics committee (Saveetha University: SRB/SDMDS15ODS1) and followed the CONSORT guidelines—www.consort-statement.org.
Sample Size Determination
All subjects were treated according to the Helsinki Declaration. Based on a similar study conducted by Nowicka et al.,5 sample size estimation was calculated using priori by the G*power 3.1.2 software with 0.90 power and p ≤ 0.05. After initial screening of 134 teeth in patients, 102 intact and periodontally healthy maxillary and mandibular premolar teeth indicated for orthodontic extraction of patients aged between 13 years and 30 years were selected for the study. The rationale behind using this particular age group of patients was because of scientific evidence that states increased probability of coronal pulp stones in patients above 30 years of age.29
Methods
Prior to the investigation, a written informed consent was obtained from all the patients involved in the clinical trial after a detailed explanation of the rationale, clinical procedure and potential risks associated with the study. After a detailed case history and clinical examination of each patient, standard intraoral radiographs and intraoral pictures of the tooth involved in the study were taken in order to ensure absence of trauma, caries, or periapical and periodontal lesions.
Randomization was done well in advance by a third person, not related to the study. Using block randomization, the 102 samples were randomly allocated as 34 samples each to the groups—group I: MTA Angelus (Angelus Indústria de Produtos Odontológicos, Brazil); group II: Biodentine (Septodont, Saint-Maur-des-Fosses, France); group III: Propolis powder (Hitech Naturals Pvt. Ltd., New Delhi, India). The sequentially numbered opaque sealed envelopes (SNOSE) method was employed for allocation concealment. The patient and the outcome assessor were blinded to the groups of materials used but blinding of the single operator and data analyst was not possible in this type of clinical trial, making this a double-blinded study.30 The cold test (Roeko Endo Frost, Coltene, Altstätten, Switzerland) and electric pulp tester (Digitest Pulp Vitality Tester, Parkell Inc., New York) were performed prior the procedure on all sample teeth to assess the sensibility status of the pulp. Preoperative radiographs were taken using a position-indicating device and Vista-Scan Periapical Radiographic Plate (Dürr Dental, United Kingdom) via the paralleling technique. The radiographic plate also known as photostimulable phosphor plate (PSP) was used for the study in order to obtain higher-quality images. The patients were asked to rinse their mouth with 0.2% chlorhexidine solution (Hexidine, ICPA Health Products Ltd., India). Local anesthesia (2% lidocaine and 1:100,000 adrenaline; pharmacaine, A/Pharmax Pvt. Ltd., India) was administered and rubber dam isolation was done. A class 1 occlusal cavity was prepared using a sterile No. 330 high-speed bur with water spray. A sterile ½ round bur (0.6 mm diameter) was used to intentionally expose the buccal pulp horn. The subsequent bleeding was arrested using a cotton pellet saturated with saline. New burs were used during each operation. Depending on the randomly allotted group, the teeth were treated as follows:
Group I
MTA Angelus (Angelus, Londrina, Brazil) was prepared according to manufacturer’s instructions to obtain a cement with a putty-like consistency.
Group II
The Biodentine capsule was triturated according to the manufacturer’s instructions using an amalgamator for 30 seconds to obtain a homogeneous mix of putty consistency.
Group III
About 1½ g of standardized propolis extract powder (Hitech Natural Products LTD, New Delhi) was mixed with 70% ethanol solution to form a thick mix with a packable consistency.
The chosen material was very carefully placed over the exposed pulp horn using an MTA carrier and condensed lightly with a moist cotton. A thick mix of interim restorative material (IRM) was packed tightly over the cavity.
Immediate postoperative radiographs were taken for records. The patients were recalled after 12 weeks. All teeth were tested for cold test and electric pulp tester to assess if the pulp had maintained its sensibility through the follow-up weeks. Post-follow-up radiographs were taken using the same technique as mentioned above. The teeth were then sent for extraction and histopathological evaluation.
Histopathologic Evaluation
After fixation in a 10% formalin solution, the specimens were demineralized using the decalcification process and embedded in paraffin. The 2–3 μm thickness sections were cut serially in a buccolingual plane. The prepared slides were analyzed under a high-magnification microscope to confirm the presence of a hemogeneous dentin bridge (Fig. 1). The amount of hard tissue formed at the interface of the capping material and pulp was determined according to the modified criteria by Faraco et al.31 and Medina et al.32 In our study, we used three parameters of the mentioned histologic scoring system. Each section was scored from 1 to 4, with 1 representing the most desired results and 4 representing the least desired result. The thickness of the dentinal bridge was measured at the thickest, thinnest, and midmost point areas of the continuous dentin bridge. The average of the three values was calculated and the grades were tabulated.
Statistical Analysis
The statistical analysis was performed using a statistical software program Windows, Version 20.0 (SPSS). To compare the continuity, morphology, and thickness of the dentinal bridge between the groups, the Chi-square test was applied. The significance level was fixed as 5% (α = 0.05).
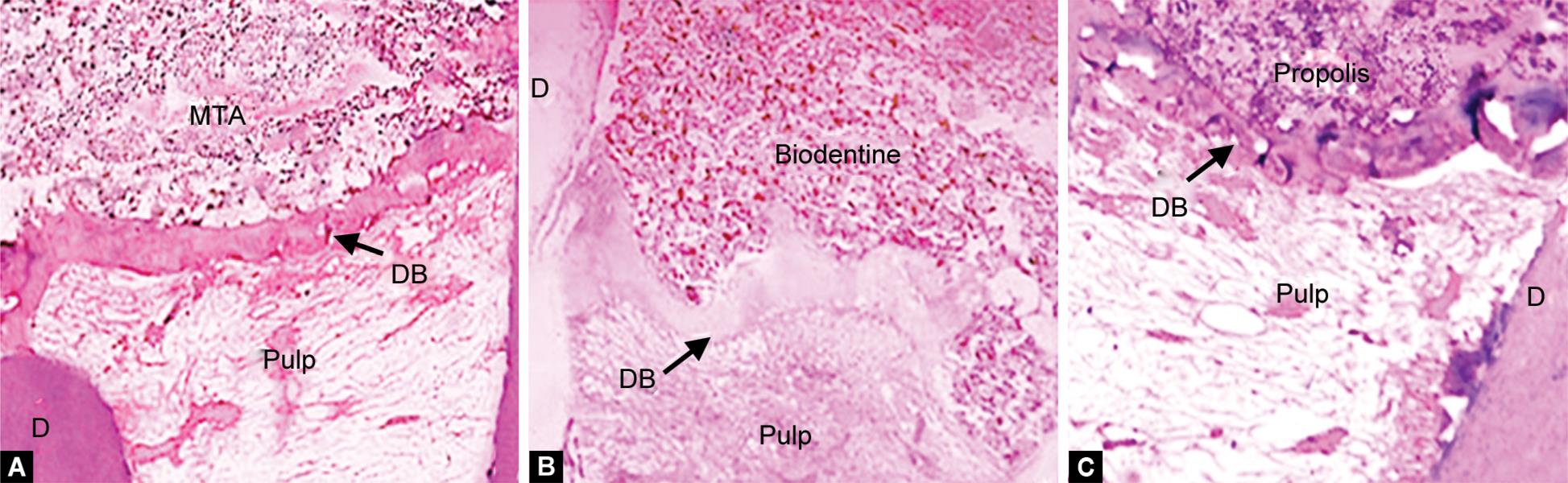
Figs 1A to C: Image representing histopathological section (hematoxylin-eosin stain; magnification 10×): (A) Group I—MTA; (B) Group II—Biodentine; (C) Group III—Propolis. D, dentin; DB, dentin bridge; MTA, mineral trioxide aggregate
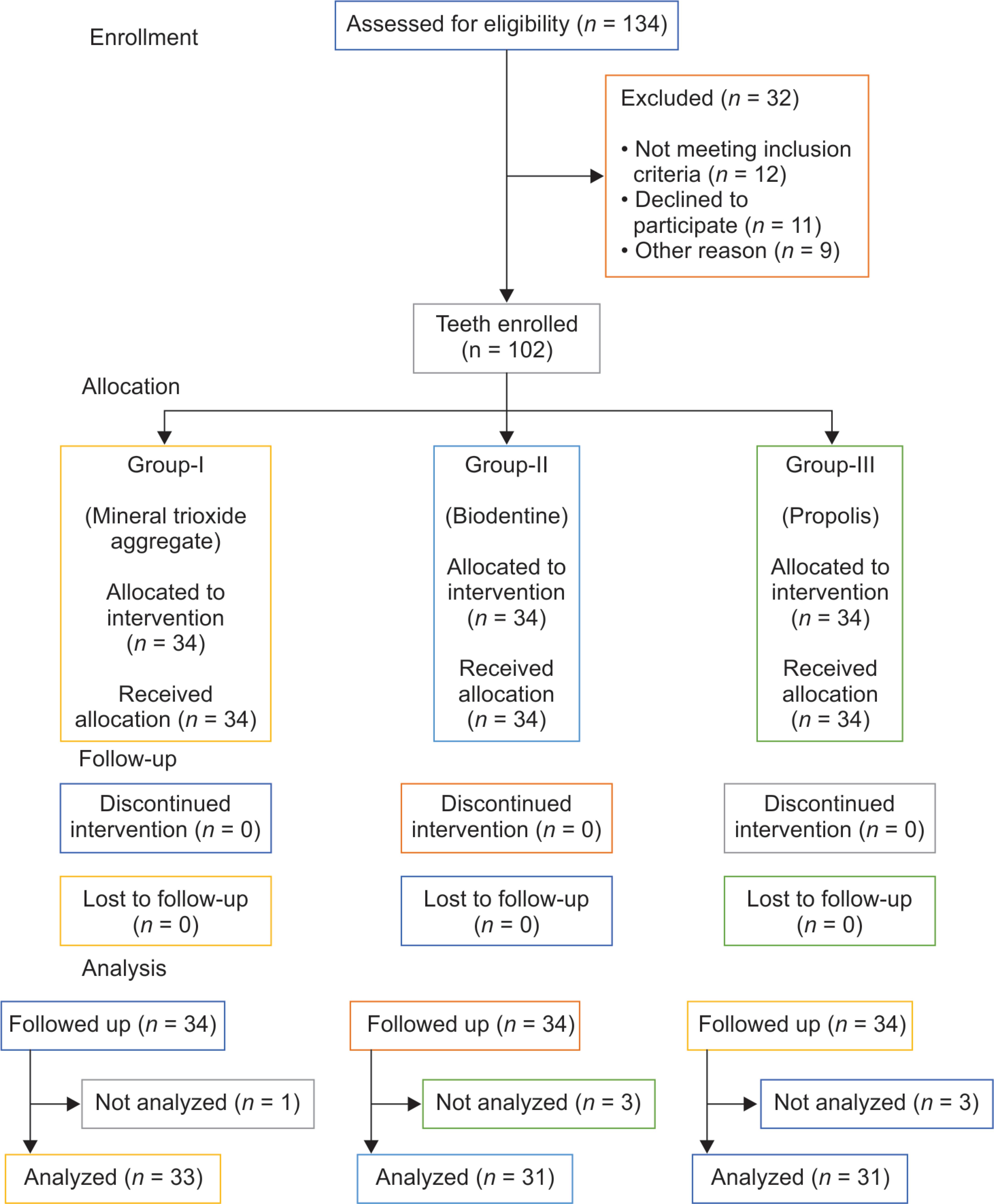
Flowchart 1: Image representing CONSORT participants flow diagram
RESULTS
As per CONSORT guidelines, Flowchart 1 depicts a self-explanatory participant flow chart. All samples retained their pulp sensibilities over the follow-up period (Fig. 2). No periapical pathologies were revealed radiographically before extraction. The dentin bridge was visibly formed in all samples of teeth. Though we did not have any loss to follow-up, a few of the samples were eliminated and not analyzed due to technical errors during histological sectioning. The number of samples finally analyzed were group I (MTA): 33; group II (Biodentine): 31; group III (Propolis): 31. Histologic evaluation of teeth showed that though group II exhibited better results than group I in all three variables (continuity, morphology, and thickness), the difference in values was statistically insignificant. The values obtained from group III were statistically significant (p %3C; 0.05) when compared to groups I and II for all three variables (Figs 3 to 5). The complete dentin bridge was observed in 26 teeth in MTA, 29 teeth in Biodentine, and 6 teeth in Propolis (Table 1). In 90.9% of group I, 93.5% of group II, and 67.7% of group III, the dentin bridge formed was associated with dentin-like hard tissue (Table 2). The mean thicknesses of the hard tissue calculated in Biodentine and MTA groups were 234.43 and 229.81 μm, respectively. The mean value obtained from the Propolis group was 129.66 μm. More than 60% of the specimens of groups I and II exhibited mean thickness of above 250 μm while on the other hand, more than 50% of group III exhibited mean thickness less than 100 μm (Table 3).
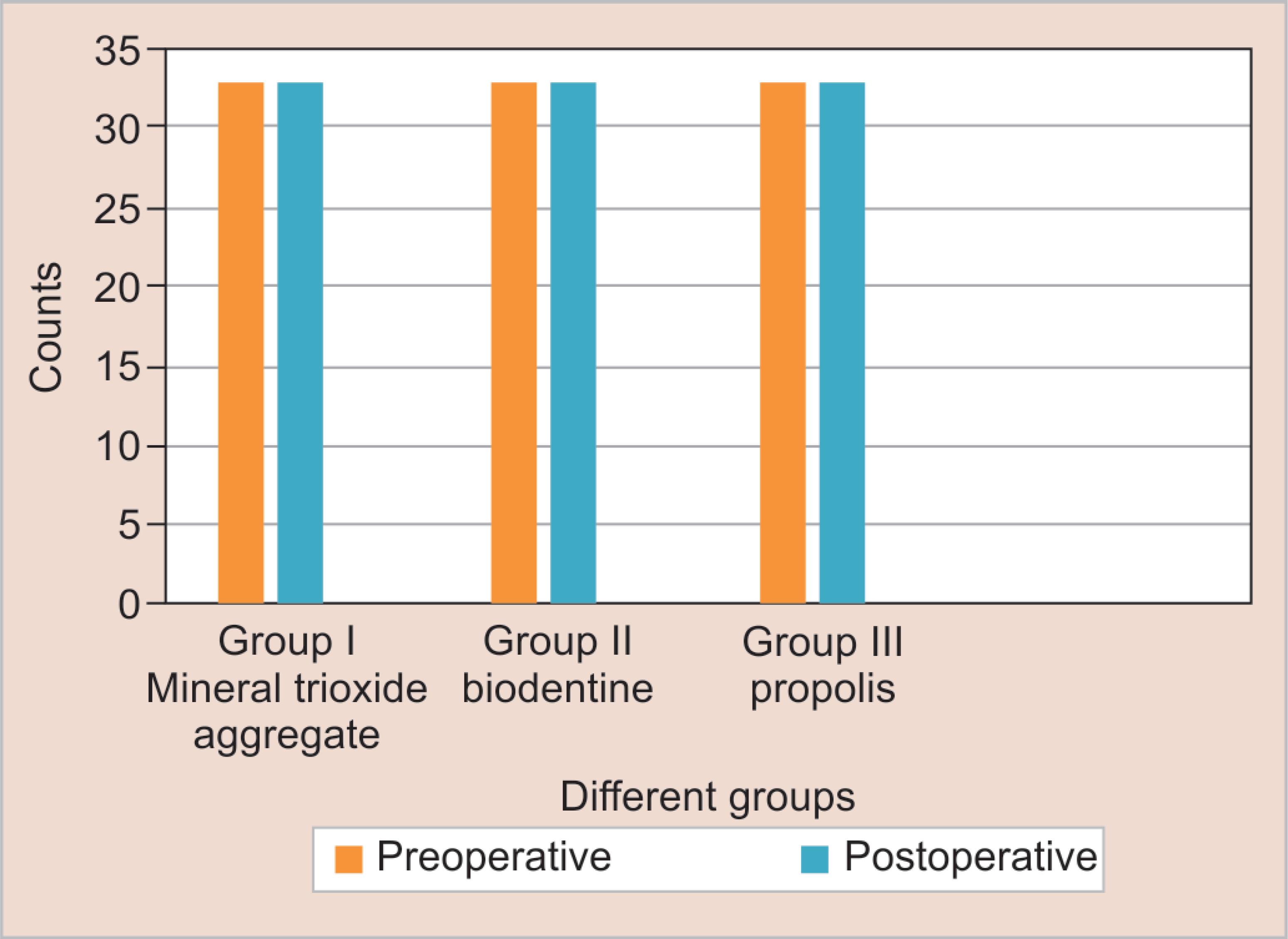
Fig. 2: Association between preoperative and postoperative pulp sensibility between groups I, II and III. The association was analyzed using the Chi-square test and was found to be statistically not significant (p %3E; 0.05)
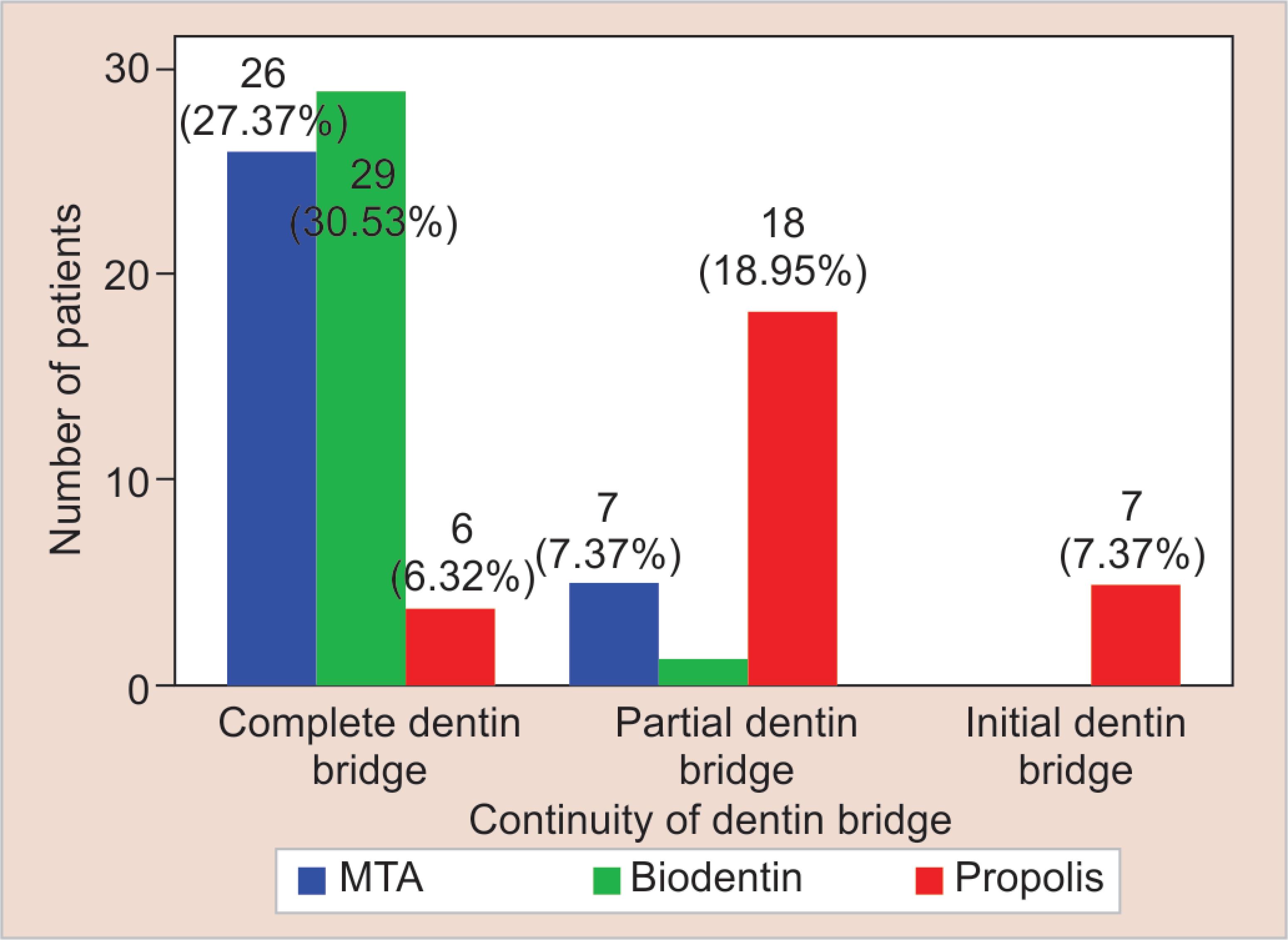
Fig. 3: Comparison of continuity of dentin bridge between mineral trioxide aggregate (MTA), Biodentine, and Propolis

Fig. 4: Comparison of morphology of dentin bridge between mineral trioxide aggregate (MTA), Biodentine, and Propolis
DISCUSSION
In our study, the primary outcome measure was pulp sensibility using the cold test and EPT and the secondary outcome measure was dentin bridge formation, which was assessed using a scoring system as mentioned earlier. All the samples positively maintained pulp sensibility and also showed visible dentin bridge histologically. The major advantage of selecting orthodontic patients whose premolars were indicated for extraction was that we had 0% loss to follow-up. Additionally, using caries-free teeth with healthy odontoblastic cells helped us analyze the true reactionary process of the test materials with the exposed site of pulp and dentin.
Mineral trioxide aggregate and Biodentine have time and again proved their potency as pulp-capping agents. In various reviews between MTA and Biodentine, researchers have found statistically insignificant results, but they usually conclude by stating lack of accountable evidence due to various limitations in the clinical trials.33,34 Nowicka et al. conducted a study similar to our clinical trial on intact third molars using the same histological grading system as ours in which they also observed statistically no difference in Biodentine and MTA.35
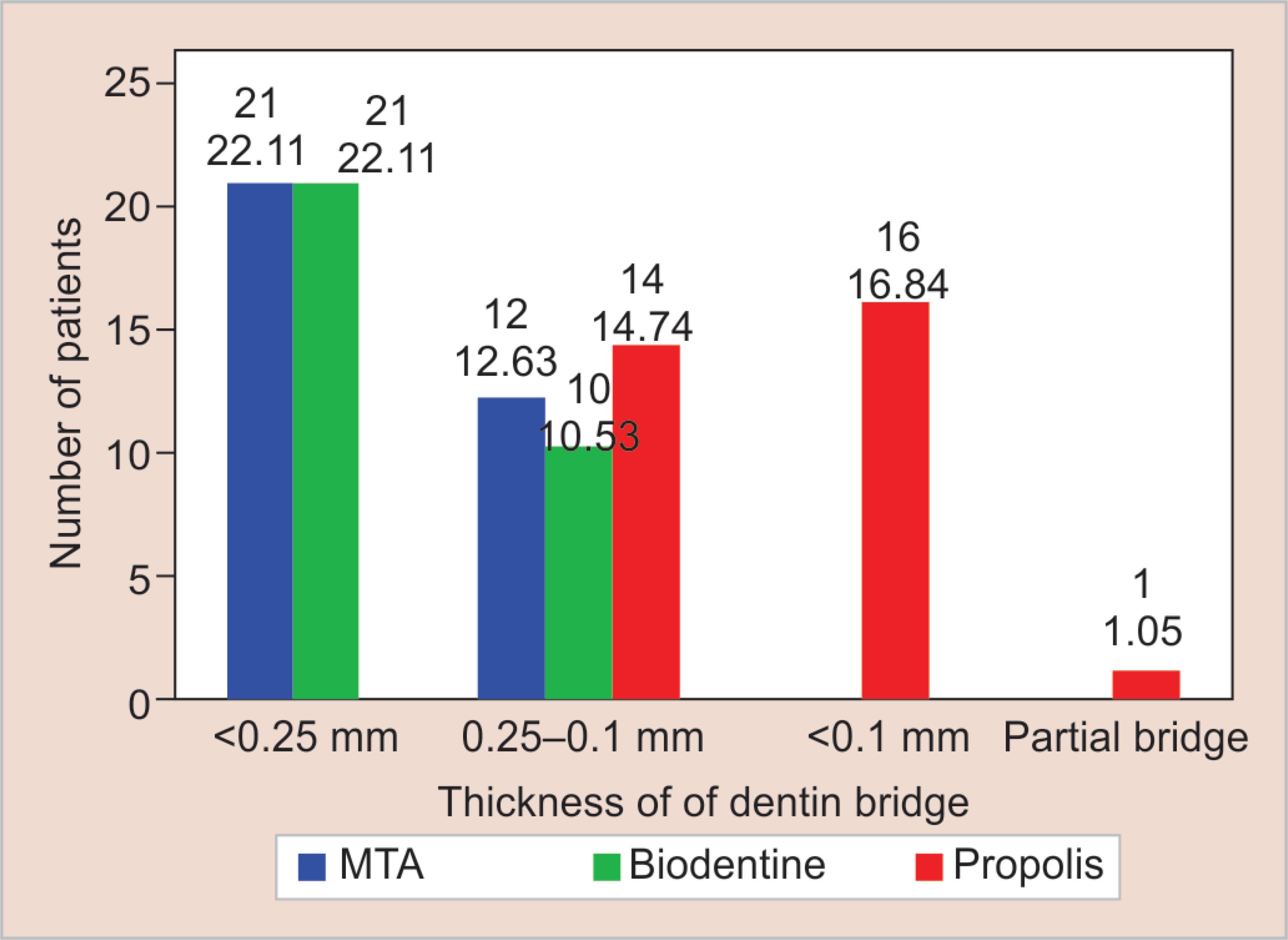
Fig. 5: Comparison of thickness of dentin bridge between mineral trioxide aggregate (MTA), Biodentine, and Propolis
Despite overcoming the shortcomings of these trials, like following CONSORT guidelines including adequate sample size and duration of study, we also observed similar results., i.e., though Biodentine showed superior values in terms of the three parameters chosen for our study, the values when compared to MTA were statistically insignificant. Previously conducted similar studies have observed superior mechanical properties of Biodentine than MTA.5,34,35 In the current study as well, we found handling of Biodentine to be much easier than MTA, which was time-consuming and technically complicated. On the other hand, preparing Biodentine requires a triturator unlike in case of MTA. According to our study, Biodentine establishes itself as an equally effective pulp-capping agent as MTA and it can be very well replaced by the conventional slow-setting MTA in cases involving vital pulp therapy because of its better physical properties. Further studies need to be conducted to establish Biodentine as a panacea in dentistry.
| Groups | Total | Chi-square | p value | ||||
|---|---|---|---|---|---|---|---|
| I | II | III | |||||
| Complete dentin bridge | N | 26 | 29 | 6 | 61 | 44.866 | 0.000 |
| % | 78.8 | 93.5 | 19.4 | 64.2 | |||
| Partial dentin bridge | N | 7 | 2 | 18 | 27 | ||
| % | 21.2 | 6.5 | 58.1 | 28.4 | |||
| Initial dentin bridge | N | 0 | 0 | 7 | 7 | ||
| % | 0.0 | 0.0 | 22.6 | 7.4 | |||
| Total | N | 33 | 31 | 31 | 95 | ||
| % | 100.0 | 100.0 | 100.0 | 100.0 | |||
| Groups | Total | Chi-square | p value | ||||
|---|---|---|---|---|---|---|---|
| I | II | III | |||||
| Dentin associated with dentin-like hard tissue | N | 30 | 29 | 21 | 80 | 10.016 | 0.04 |
| % | 90.9 | 93.5 | 67.7 | 84.2 | |||
| Only irregular hard tissue deposition | N | 3 | 2 | 9 | 14 | ||
| % | 9.1 | 6.5 | 29.0 | 14.7 | |||
| Only a thin layer of hard tissue deposition | N | 0 | 0 | 1 | 1 | ||
| % | 0.0 | 0.0 | 3.2 | 1.1 | |||
| Total | N | 33 | 31 | 31 | 95 | ||
| % | 100.0 | 100.0 | 100.0 | 100.0 | |||
| Groups | Total | Chi-square | p value | ||||
|---|---|---|---|---|---|---|---|
| I | II | III | |||||
| >250 μm | N | 21 | 21 | 0 | 42 | 61.780 | 0.000 |
| % | 63.6 | 67.7 | 0.0 | 44.2 | |||
| 250-100 μm | N | 12 | 10 | 14 | 34 | ||
| % | 36.4 | 32.3 | 35.5 | 34.7 | |||
| <100 μm | N | 0 | 0 | 16 | 16 | ||
| % | 0.0 | 0.0 | 51.6 | 16.8 | |||
| Partial bridge | N | 0 | 0 | 1 | 1 | ||
| % | 0.0 | 0.0 | 3.2 | 1.2 | |||
| Total | N | 33 | 31 | 31 | 95 | ||
| % | 100.0 | 100.0 | 100.0 | 100.0 | |||
Furthermore, in our clinical trial, it was observed that propolis was well-tolerated by the pulpal tissues and it successfully induced dentin bridges in all the teeth of its group. When compared to Biodentine and MTA, the continuity, morphology, and thickness shown in teeth capped with Propolis had significantly less values. The reason for this result can be assumed to be the difference in the material composition. Propolis has a broad spectrum of biological and pharmacological properties such as antioxidant, anti-inflammatory, and antibacterial. It contains a rich variety of potent terpene, benzoic, caffeic, cinnamic, aromatic aldehyde, phenolic acid, vitamins A, B complex, C, E, and minerals along with high flavonoid content, which catalyzes wound healing on an exposed dental pulp and improves tissue regeneration.36 But it has been observed that cement groups like MTA and Biodentine induce apatite precipitates and generate higher amounts of remineralizing elements at a much faster rate.37
It needs to be brought to notice that even with statistically significant difference in values of the two groups compared to Propolis, none of the treated teeth of this group showed loss in sensibility or absence of dentin bridge. Also, other histological studies on Propolis as pulp-capping agents have shown its superior tolerance to pulpal tissues due to its anti-inflammatory properties.38–40 On the other hand, pulp-capping agents with high pH function by causing initial pulpal inflammation and necrosis of the superior layer of the pulp, later leading to bridge formation, sometimes even leading to a void formation between the material and the bridge,41 which, in the long-term, can be cause of concern. Additionally, it has been discussed by many researchers that reparative dentin following pulp capping involves formation of TGF-β1 growth factor (transforming growth factor). Propolis has shown to stimulate TGF-β1 resulting in odontoblast-like cell differentiation.42,43
One of the limitations in our study was the elimination of a few samples due to errors during histological sectioning. In future studies, more efficient and less-technique sensitive methods for histological analysis can be employed. Also, our study took into consideration three parameters—continuity, morphology, and thickness, pertaining to the quality and quantity of hard tissue bridges formed. Studies need to be carried out to evaluate the other parameters of the histological scoring system, which is based on evaluation of inflammatory process, odontoblastic layer, and presence of microorganisms.
According to our study and parameters assessed, Propolis effectively serves its purpose as a pulp-capping agent in cases of noncariogenic accidental traumatic exposures. Further studies need to be carried out to establish the reaction of Propolis in carious teeth, where the odontoblasts have been damaged or destroyed.
CONCLUSION
All the three materials—MTA, Biodentine and Propolis—have the ability to sustain pulp sensibility and induce dentin bridge in noncarious pulpal exposures. The quality and quantity of dentin bridge formation by MTA and Biodentine was similar to each other and superior to Propolis. Thus clinically, Biodentine has proved to be an effective pulp-capping agent and is comparable to MTA. On the other hand, Propolis being an organic material can be successfully used as a direct pulp-capping agent due to its ability to induce reparative dentin and maintain pulp sensibility for a long period of time.
ACKNOWLEDGMENTS
We would like to thank the Department of Orthodontics and the Department of Oral and Maxillo-Facial Pathology, Saveetha Dental College, and Saveetha Institute of Medical and Technical Sciences for helping us with patient selection and to process and analyze the histopathological aspect of this study, respectively.
DECLARATION OF PATIENT CONSENT
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
REFERENCES
1. Ferracane JL, Cooper PR, Smith AJ. Can interaction of materials with the dentin-pulp complex contribute to dentin regeneration? Odontology 2010;98(1):2–14. DOI: 10.1007/s10266-009-0116-5.
2. Franz FE, Holz J, Baume LJ. Ultrastructure (SEM) of dentine bridging in the human dental pulp. J Biol Buccale 1984;12(3):239–246.
3. Schröder U. Effects of calcium hydroxide-containing pulp-capping agents on pulp cell migration, proliferation, and differentiation. J Dent Res 1985;64(Spec No):541–548. DOI: 10.1177/002203458506400407.
4. Song M, Yu B, Kim S, et al. Clinical and molecular perspectives of reparative dentin formation: lessons learned from pulp-capping materials and the emerging roles of calcium. Dent Clin North Am 2017;61(1):93–110. DOI: 10.1016/j.cden.2016.08.008.
5. Nowicka A, Wilk G, Lipski M, et al. Tomographic evaluation of reparative dentin formation after direct pulp capping with ca(OH)2, MTA, biodentine, and dentin bonding system in human teeth. J Endod 2015;41(8):1234–1240. DOI: 10.1016/j.joen.2015.03.017.
6. Li Z, Cao L, Fan M, et al. Direct pulp capping with calcium hydroxide or mineral trioxide aggregate: a meta-analysis. J Endod 2015;41(9):1412–1417. DOI: 10.1016/j.joen.2015.04.012.
7. Al-Hezaimi K, Salameh Z, Al-Fouzan K, et al. Histomorphometric and micro–computed tomography analysis of pulpal response to three different pulp capping materials. J Endod 2011;37(4):507–512. DOI: 10.1016/j.joen.2010.11.001.
8. Cox CF, Sübay RK, Ostro E, et al. Tunnel defects in dentin bridges: their formation following direct pulp capping. Oper Dent 1996;21(1):4–11.
9. Nair PNR, Duncan HF, Pitt Ford TR, et al. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: a randomized controlled trial. Int Endod J 2008;41(2):128–150.
10. Asgary S, Eghbal MJ, Parirokh M, et al. A comparative study of histologic response to different pulp capping materials and a novel endodontic cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008;106(4):609–614. DOI: 10.1016/j.tripleo.2008.06.006.
11. Tran XV, Gorin C, Willig C, et al. Effect of a calcium-silicate-based restorative cement on pulp repair. J Dent Res 2012;91(12):1166–1171. DOI: 10.1177/0022034512460833.
12. Parirokh M, Asgary S, Eghbal MJ, et al. A comparative study of using a combination of calcium chloride and mineral trioxide aggregate as the pulp-capping agent on dogs’ teeth. J Endod 2011;37(6):786–788. DOI: 10.1016/j.joen.2011.03.010.
13. Dominguez MS, Witherspoon DE, Gutmann JL, et al. Histological and scanning electron microscopy assessment of various vital pulp-therapy materials. J Endod 2003;29(5):324–333. DOI: 10.1097/00004770-200305000-00003.
14. Iwamoto CE, Adachi E, Pameijer CH, et al. Clinical and histological evaluation of white ProRoot MTA in direct pulp capping. Am J Dent 2006;19(2):85–90.
15. Shen Y, Peng B, Yang Y, et al. What do different tests tell about the mechanical and biological properties of bioceramic materials? Endod Topics 2015;32(1):47–85. DOI: 10.1111/etp.12076.
16. Stringhini EJr, dos Santos MGC, Oliveira LB, et al. MTA and biodentine for primary teeth pulpotomy: a systematic review and meta-analysis of clinical trials. Clin Oral Investig 2019;23(4):1967–1976. DOI: 10.1007/s00784-018-2616-6.
17. Kaur M, Singh H, Dhillon JS, et al. MTA versus biodentine: review of literature with a comparative analysis. J Clin Diagn Res 2017;11(8):ZG01–ZG05. DOI: 10.7860/JCDR/2017/25840.10374.
18. Sudarshan R, Vijayabala GS. Role of ginger in medicine and dentistry: an interesting review article. Southeast Asian J Case Rep Rev 2012;1:66–72.
19. Subhash AV, Suneela S, Anuradha C, et al. The role of aloe vera in various fields of medicine and dentistry. J Orofac Sci 2014;6(1):5. DOI: 10.4103/0975-8844.132564.
20. Malviya J, Joshi V. Anticancer activity evaluation and some Indian medicinal plants from amarkantak Mekal Plateau Madhya Pradesh, India [internet]. Int J Curr Microbiol Appl Sci 2016;5(11):478–483. DOI: 10.20546/ijcmas.2016.511.055.
21. Ishnava KB, Chauhan JB, Barad MB. Anticariogenic and phytochemical evaluation of eucalyptus globules labill. Saudi J Biol Sci 2013;20(1):69–74. DOI: 10.1016/j.sjbs.2012.11.003.
22. Mohanty S, Ramesh S, Muralidharan NP. Antimicrobial efficacy of apple cider vinegar against enterococcus faecalis and Candida albicans: an in vitro study. J Adv Pharma Edu Res 2017;7(2):137–141.
23. Oncag O, Cogulu D, Uzel A, et al. Efficacy of propolis as an intracanal medicament against enterococcus faecalis. Gen Dent 2006;54(5):319–322.
24. Madhubala MM, Srinivasan N, Ahamed S. Comparative evaluation of propolis and triantibiotic mixture as an intracanal medicament against enterococcus faecalis. J Endod 2011;37(9):1287–1289. DOI: 10.1016/j.joen.2011.05.028.
25. Mori GG, Nunes DC, Castilho LR, et al. Propolis as storage media for avulsed teeth: microscopic and morphometric analysis in rats. Dent Traumatol 2010;26(1):80–85. DOI: 10.1111/j.1600-9657.2009.00856.x.
26. Sanghavi T, Shah N, Parekh V, et al. Evaluation and comparison of efficacy of three different storage media, coconut water, propolis, and oral rehydration solution, in maintaining the viability of periodontal ligament cells. J Conserv Dent 2013;16(1):71–74. DOI: 10.4103/0972-0707.105303.
27. Al-Haj Ali SN. In vitro toxicity of propolis in comparison with other primary teeth pulpotomy agents on human fibroblasts. J Investig Clin Dent 2016;7(3):308–313. DOI: 10.1111/jicd.12157.
28. Ozório JEV, Carvalho LF, de OES, et al. Standardized propolis extract and calcium hydroxide as pulpotomy agents in primary pig teeth. J Dent Child 2012;;79(2):53–58.
29. Goga R, Chandler NP, Oginni AO. Pulp stones: a review [internet]. Int Endod J 2008;41(6):457–468. DOI: 10.1111/j.1365-2591.2008.01374.x.
30. Renjith V. Blinding in randomized controlled trials: what researchers need to know? Manipal J Nurs Health Sci (MJNHS) 2017;3(1):45–50.
31. Faraco IMJr, Holland R. Response of the pulp of dogs to capping with mineral trioxide aggregate or a calcium hydroxide cement. Dent Traumatol 2001;17(4):163–166. DOI: 10.1034/j.1600-9657.2001.170405.x.
32. Medina VO3rd, Shinkai K, Shirono M, et al. Histopathologic study on pulp response to single-bottle and self-etching adhesive systems. Oper Dent 2002;27(4):330–342.
33. Kang MK. Endodontics: Clinical and Scientific Updates, an Issue of Dental Clinics of North America, E-Book. Elsevier Health Sciences; 2016. p. 193.
34. Kunert M, Szymanska ML. Bio-inductive materials in direct and indirect pulp capping - a review article. Materials 2020;13(5):1204–1224. DOI: 10.3390/ma13051204.
35. Nowicka A, Lipski M, Parafiniuk M, et al. Response of human dental pulp capped with biodentine and MTA. J Endod 2013;39(6):743–747. DOI: 10.1016/j.joen.2013.01.005.
36. Sabir A. The healing actions of propolis on direct pulp capping treatment: a review. J Dentomaxillofac Sci 2016;1(1):188. DOI: 10.15562/jdmfs.v1i1.145.
37. Li X, De Munck J, Van Landuyt K, et al. How effectively do hydraulic calcium-silicate cements re-mineralize demineralized dentin. Dent Mater 2017;33(4):434–445. DOI: 10.1016/j.dental.2017.01.015.
38. Inoki R, Kudo T, Olgart LM. Dynamic Aspects of Dental Pulp: Molecular biology, pharmacology and pathophysiology. Springer Science and Business Media; 2012. p. 508.
39. Junior ES, Stringhini Junior E, Vitcel MEB, et al. Evidence of pulpotomy in primary teeth comparing MTA, calcium hydroxide, ferric sulphate, and electrosurgery with formocresol [internet]. Eur Archi Paediat Dent 2015;16(4):303–312. DOI: 10.1007/s40368-015-0174-z.
40. Taneja S, Singh A. Evaluation of effectiveness of calcium hydroxide and MTA as pulpotomy agents in permanent teeth: a meta-analysis. Pediat Dent J 2019;29(2):90–96. DOI: 10.1016/j.pdj.2019.04.001.
41. Jalan AL, Warhadpande MM, Dakshindas DM. A comparison of human dental pulp response to calcium hydroxide and biodentine as direct pulp-capping agents. J Conserv Dent 2017;20(2):129–133. DOI: 10.4103/0972-0707.212247.
42. Tziafas D. The future role of molecular approach to pulp-dentinal regeneration. Caries Res 2004;38(3):314–320. DOI: 10.1159/000077771.
43. Ansorge A, Reinhold D, Lendeckel U. Propolis and some of its constituents down-regulate DNA synthesis and inflammatory cytokine production but induce TGF-ß1 production of human immune cells. Z Naturforsch 2003;58(7-8):580–589. DOI: 10.1515/znc-2003-7-823.
________________________
© The Author(s). 2020 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.