CASE REPORT | https://doi.org/10.5005/jp-journals-10015-1738 |
Multilayered Platelet-rich Fibrin as a Barrier Membrane in Guided Bone Regeneration with Simultaneous Implant Placement: A 3-year Follow-up
1–4Department of Prosthodontics, Faculty of Dental Sciences, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India
5Department of OMFS, Faculty of Dental Sciences, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India; Department of Dentistry, Kalpana Chawla Government Medical College, Karnal, Haryana, India
Corresponding Author: Aditi Priya, Department of Prosthodontics, Faculty of Dental Sciences, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India, Phone: +91 9554860007, e-mail: aditi.imsbhu.priya@gmail.com
How to cite this article Soni R, Priya A, Yadav H, et al. Multilayered Platelet-rich Fibrin as a Barrier Membrane in Guided Bone Regeneration with Simultaneous Implant Placement: A 3-year Follow-up. World J Dent 2020;11(4):328–331.
Source of support: Nil
Conflict of interest: None
ABSTRACT
Aim: To demonstrate the use of multilayered platelet-rich fibrin (PRF) membranes as a barrier membrane in the guided bone regeneration (GBR) procedure.
Background: Ridge deformities, defects, and insufficient volume at the intended implant sites are common challenge. Simultaneous implant placement along with GBR gives benefits of reduced treatment cost, time, and need of additional surgical procedure. Platelet-rich fibrin has a versatile nature and is a regenerative tool in both hard and soft tissue surgery. This case report presented an alternative approach using PRF as a membrane (multilayered) in GBR to correct buccal wall defect in conjunction with implant placement.
Case description: A 23-year-old male was referred for rehabilitation of edentulous area concerning to 32 and 33 regions. After clinical and radiological evaluation and discussion of various treatment modalities, GBR with simultaneous implant placement was planned. Ridge augmentation was done in defect using sticky bone formed by bio-oss bone granules of particle size 1.0–2.0 mm and PRF membranes after implant placement. A proper surgical procedure was followed and postoperative follow-up was done from time to time.
Conclusion: Sufficient bone volume was achieved through GBR as shown clinically at second-stage surgery done after 4 months. Three years’ follow-up reveals stable implant prosthesis and healthy interdental papilla and gingival tissue. The multilayered PRF membrane is safe, more economical, and may be feasible as a barrier membrane and could be used in some selected scenarios.
Clinical significance: Platelet-rich fibrin membranes can act as resorbable barrier and allow faster healing, improve bone formation, provide soft tissue regeneration, and improve the soft tissue profile.
Keywords: Dental implant, Guided bone regeneration, Platelet-rich fibrin.
BACKGROUND
Extensive loss of the alveolar bone when encountered presents a challenge for reconstruction. Either simultaneous implant placement is done or not, the guided bone regeneration (GBR) procedure should be done when bone augmentation is needed. Membranes used with GBR fall under two main categories. They are either resorbable membranes or nonresorbable membranes. The main disadvantage of nonresorbable membrane is membrane exposure, which may result in bacterial contamination and early removal of the membrane.1,2
To avoid these problems, clinicians are advocating the benefits of using biodegradable barriers. Platelet-rich fibrin (PFR) is a second generation of the platelet derivative, which is prepared in single step and does not require any additives.3 It provides a fibrin matrix enriched with platelets, leukocytes, and growth factors.4 The fibrin network provides efficient cell proliferation, migration, and acts as a scaffold for tissue regeneration and restoration of bony defects.5
The platelet-rich fibrin (PRF) production process requires no anticoagulants during blood withdrawal and no materials are needed to be added for platelet activation or fibrin polymerization. Platelet-rich fibrin releases an array of growth factors, such as PDGF, TGF β-1, VEGF, EGF, FGF, etc. It is reported that the growth factors (GFs) are sustainably released for at least 1 week up to 28 days.6 This slow and sustainable release of GFs allows the PRF membrane to help in a faster wound healing process and due to its strong fibrin matrix, it acts as a natural barrier in guided tissue regeneration.5
CASE DESCRIPTION
A 23-year-old male in good general health, nonsmoker, and with adequate oral hygiene was referred to the Outpatient Department of Prosthodontics and Crown and Bridge of our faculty for the rehabilitation of lost teeth concerning to 32 and 33 regions. Initial radiographic evaluation by orthopantomography (OPG) revealed defect area w.r.t. to 32 and 33 region (Fig. 1). Different treatment modalities were properly discussed with the patient and his attendees. Placement of implant and the GBR procedure using PRF membranes were planned at the same sitting.
After diagnostic workout, an informed consent was taken before the surgical procedure and initial periodontal therapy was done. Surgical area was scrubbed by betadine scrub and the patient was draped. The surgical site was anesthetized by inferior alveolar nerve block on both sides with local infiltration of 2% lignocaine with 1:80,000 adrenaline. After adequate anesthesia was achieved, crestal incision was done on the edentulous region and the incision was continued as the crevicular incision on both sides w.r.t. tooth 31, 41 and 34, 35 and then releasing incision was given. After full-thickness mucoperiosteal flap reflection, buccal plate defect was seen, which was thoroughly debrided and irrigated with saline (Fig. 2A). Consequently, implants (double piece, ADIN; Touareg - S, 3.75 × 13 mm w.r.t. tooth 32 and 4.2 × 16 mm w.r.t. tooth 33) were placed w.r.t. 32 and 33 region (Fig. 2B). In between the surgical procedure, a chairside procedure for fabrication of A-PRF and I-PRF was carried out. Two silica-coated red cap tubes of 10 mL without anticoagulant were used for A-PRF membrane formation and two yellow cap tubes of 10 mL were used for obtaining I-PRF. Patient’s blood was withdrawn directly into tubes by vacutainers, and tubes were transferred to the centrifugation machine (DUO Quattro Centrifuge, Nice, France) for obtaining I-PRF and A-PRF one by one. For obtaining I-PRF, centrifugation was done at 700 revolutions per minute (rpm) for 3 minutes; the yellow upper part was obtained by a syringe and mixed with the particulate bone graft (Bio-oss bone granules; natural bovine bone graft, particle size 1.0–2.0 mm) to form sticky bone for improved handling to improve graft stability.7–9 For obtaining A-PRF, centrifugation was done at 1300 rpm for 8 minutes.9 The PRF clot was retrieved from the tubes, placed in the PRF box, and transformed into a membrane. The sticky bone was placed into the defect and over the exposed implant threads and multilayered PRF membranes were used as a barrier membrane (Fig. 3). Primary closure was done by interrupted sutures (3-0 silk suture). Postoperative instructions were given and the patient medications were prescribed for 7 days. The patient was recalled after 7 days for removal of suture and further evaluation.
After 4 months at the second-stage surgery, a surgical reentry was performed and it was seen that the buccal bone was satisfactorily regenerated at 32 and 33 regions, covering all implant threads (Fig. 4A). Slight cleft was visible over the newly formed bone (Fig. 4A), so additional grafting was done by bio-oss granules (Fig. 4B). Healing abutments were placed and sutures were performed. The medication was prescribed and oral hygiene measures were explained. After second-stage surgery when healing was obtained, abutments were prepared and final prosthesis (zirconia crown) was delivered (Fig. 5A). Stability of implants was checked at the time of delivery of prosthesis. Regular follow-up was done; implant prosthesis was stable and functional at 3rd-year follow-up with healthy gingival tissue and good papillary adaptation (Fig. 5B). Orthopantomography revealed favorable bone regeneration and successful implant outcomes with the absence of peri-implant defects (Fig. 6).
DISCUSSION
During the GBR procedure with simultaneous implant placement, it is necessary to create a space between the implant and surrounding soft tissues and it should be maintained for an appropriate period of time to prevent migration of nonosteogenic tissues into the concerned area and thus ensuring osteogenesis. In addition to space maintenance and prevention of migration of nonosteogenic tissues, the membrane also plays a role in graft stabilization.1,2
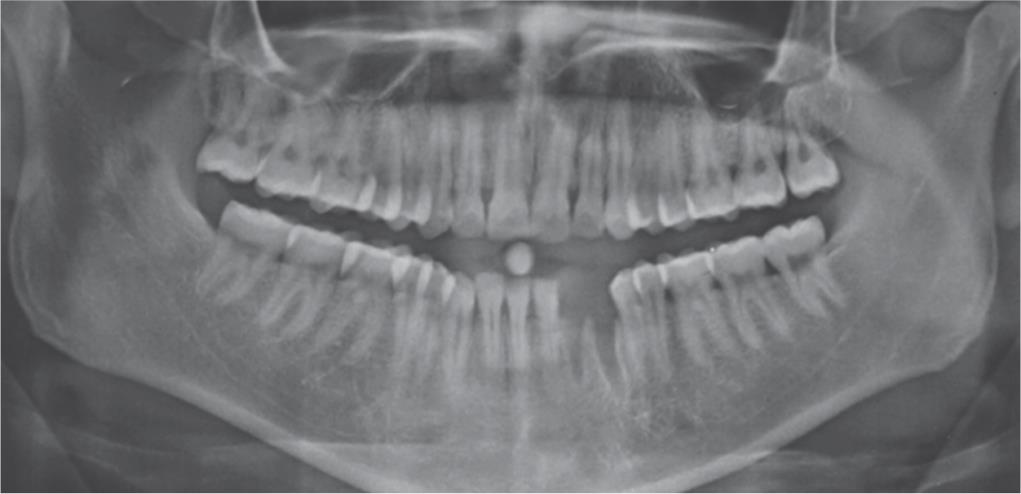
Fig. 1: Preoperative OPG X-ray showing defect
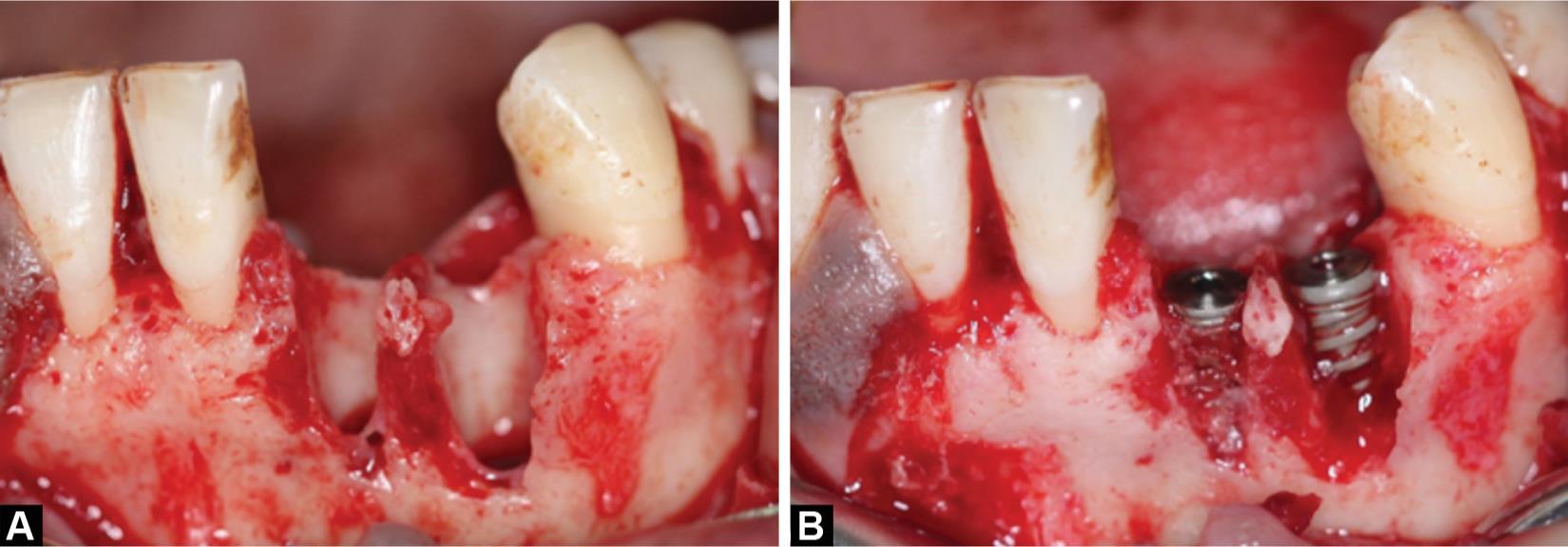
Figs 2A and B: Intraoral view: (A) Defect area before implant placement; (B) Defect area after implant placement
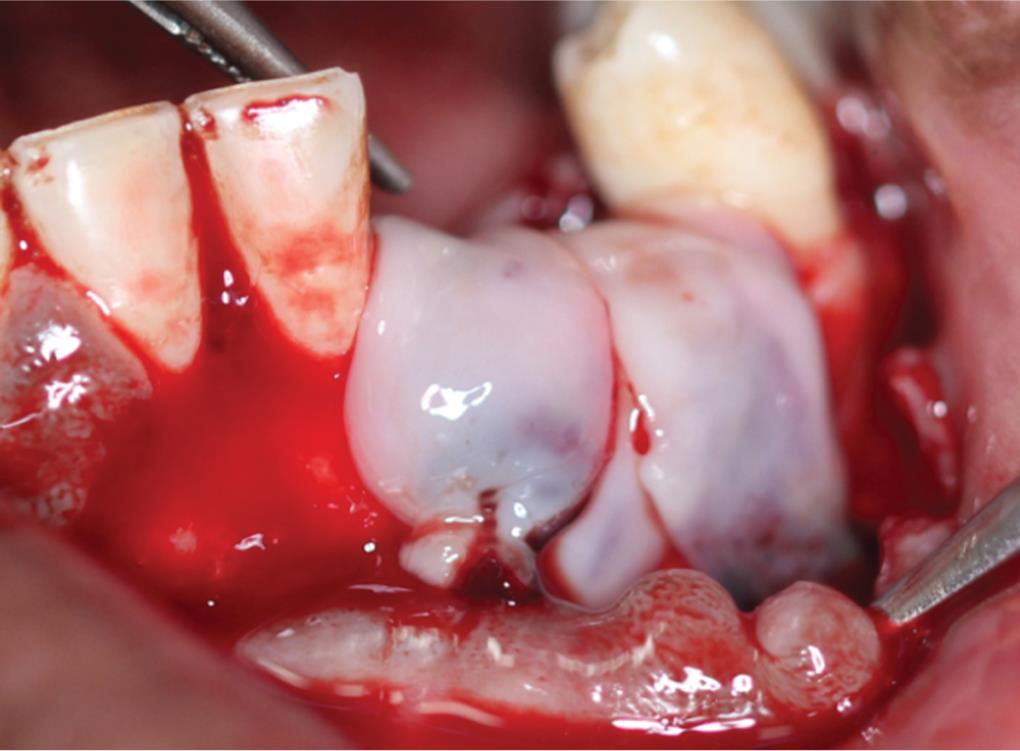
Fig. 3: Multilayered PRF membranes covering the bone graft

Figs 4A and B: Intraoral view: (A) Regenerated buccal bone with slight cleft; (B) Additional bone graft applied over the cleft

Figs 5A and B: Intraoral view: (A) After prosthesis delivery; (B) Stable and functional implant prosthesis at 3rd year follow-up with healthy gingival tissue and good papillary adaptation

Fig. 6: Orthopantomography showing implant prosthesis with favorable bone regeneration
Platelet-rich fibrin is a second-generation platelet concentrate and is considered as fibrin biomaterial, which acts as a reservoir of growth factors.3–5 It has a natural fibrin framework, which favors microvascularization and promotes migration of cells to its surface. Furthermore, this fibrin matrix contains leukocytes and promotes their migration. Platelet-rich fibrin serves as a scaffold to stem cells due to its fibrin matrix. Leukocytic cytokines are trapped within the fibrin meshes of PRF and is slowly released. Platelet-rich fibrin releases an array of GFs, such as PDGF, TGF β-1,VEGF, EGF, FGF, etc.10,11 Platelet-rich fibrin is known to release GFs for at least 7 days. Platelet-rich fibrin assists in bone formation by influencing expression of osteoblastic gene.12 Platelet-rich fibrin helps in healing and bone regeneration by generating platelet and leukocyte components, which play important roles in immune response and angiogenesis.3–5,13
In this present case study, a multilayered PRF membrane was used to cover the bone defect and bone graft placed in it. The multilayered PRF membranes used aid as a covering for the graft material and provided a barrier effect for new bone regeneration. Despite of bone defect, a good bone volume was obtained after the application of bone graft and multilayered PRF membranes. After 4 months, gingival tissues are in good health and showed good maturation. In a study done by Hafez et al.,13 the PRF membrane was utilized to cover the peri-implant defect in immediate implants in the maxillary anterior region and clinical and radiographic evaluation was performed to evaluate soft tissue healing and crestal bone stability, which showed that PRF provided adequate soft tissue coverage over the immediate implants and strengthen bone stability.13 The result of our present case study showed that the application of multilayered PRF membranes may be successful to serve as a resorbable barrier membrane when performing GBR procedures. These findings are also consistent with the study by Gassling et al.14 who evaluated the vital bone formation and bone quality in maxillary sinus floor augmentation after placement of two different absorbable membranes (PRF vs collagen) at the site of the lateral sinus wall osteotomy.14 He demonstrated that the bone quality and bone formation was the same for both the groups. Another randomized controlled study by Mehta et al.15 compared PRF and the collagen membrane in the treatment of furcation defects in which the PRF group showed better results both clinically and radiographically.15 From the abovementioned findings, we can extrapolate that the use of the PRF membrane as a barrier membrane in the GBR approach is inexpensive, easily obtained, and without any risk of foreign body reactions. We can summarize the advantages of the PRF membrane over the collagen membrane as follows:
- Autogenous source.
- No additives or anticoagulants required in fabrication.
- Acts as a reservoir of growth factors.
- After compression into a membrane form, PRF have good consistency to be used as a barrier membrane.
- Inexpensive and easy to prepare.
- Peri-implant hard and soft tissue enhancement.
CONCLUSION
The results of our case study showed that multilayered PRF membranes can be adequately and successfully used over immediately placed implants and in GBR. The use of PRF as membranes can induce and improve bone formation and also provide good esthetic results by improving the soft tissue profile. The approach of using multilayered PRF membranes as a resorbable membrane for GBR as described in this case report is safe, more economical, and could be used in some selected scenarios.
CLINICAL SIGNIFICANCE
The use of PRF as a membrane in GBR can act as a resorbable barrier, allowing faster healing, improving bone formation, providing soft tissue regeneration, and improving the soft tissue profile.
REFERENCES
1. Dimitriou R, Mataliotakis G, Maria Calori G, et al. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: Current experimental and clinical evidence. BMC Med 2012;10(1):1–24. DOI: 10.1186/1741-7015-10-81.
2. Nagappa R, Elzer AS, Younis SF, et al. Surgical procedure for guided bone regeneration using resorbable membrane barrier for ridge augmentation in successful implant placement. J Indian Acad Dent Spec Res 2015;2(2):70–75. DOI: 10.4103/2229-3019.177931.
3. Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101(3):e45–e50. DOI: 10.1016/j.tripleo.2005.07.009.
4. Peck Thabit M, Marnewick J, Stephen L. Alveolar ridge preservation using leukocyte and platelet rich fibrin: a report of a case. Case Rep Dent 2011;2011:345048. DOI: 10.1155/2011/345048.
5. Choukroun J, Diss A, Simonpieri A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101(3):e56–e60. DOI: 10.1016/j.tripleo.2005.07.011.
6. He L, Lin Y, Hu X, et al. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;108(5):707–713. DOI: 10.1016/j.tripleo.2009.06.044.
7. Kobayashi E, Flückiger L, Fujioka-Kobayashi M, et al. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig 2016;20(9):2353–2360. DOI: 10.1007/s00784-016-1719-1.
8. Fujioka-Kobayashi M, Miron RJ, Hernandez M, et al. Optimized platelet-rich fibrin with the low-speed concept: growth factor release, biocompatibility, and cellular response. J Periodontol 2017;88(1):112–121. DOI: 10.1902/jop.2016.160443.
9. Dohan Ehrenfest DM, Del Corso M, Kang BS, et al. The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors and fibrin architecture of a leukocyte- and platelet-rich fibrin (L-PRF) clot and membrane. Part 3: comparison of the growth factors content and slow release between the original L-PRF and the modified A-PRF (advanced platelet-rich fibrin) membranes. POSEIDO 2014;2:155–166.
10. Appel TR, Potzsch B, Muller J, et al. Comparison of three different preparations of platelet concentrates for growth factor enrichment. Clin Oral Implants Res 2002;13(5):522–528. DOI: 10.1034/j.1600-0501.2002.130512.x.
11. Dohan Ehrenfest DM, de Peppo GM, Doglioli P, et al. Slow release of growth factors and thrombospondin-1 in Choukroun’s platelet-rich fibroin (PRF): a gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors 2009;27(1):63–69. DOI: 10.1080/08977190802636713.
12. Clipet F, Tricot S, Alno N, et al. In vitro effects of Choukroun’s platelet-rich fibrin conditioned medium on 3 different cell lines implicated in dental implantology. Implant Dent 2012;21(1):51–56. DOI: 10.1097/ID.0b013e31822b9cb4.
13. Hafez WK, Seif SA, Shawky H, et al. Platelet rich fibrin as a membrane for coverage of immediate implants: case-series study on eight patients. Tanta Dental Journal 2015;12(3):203–210. DOI: 10.1016/j.tdj.2015.05.009.
14. Gassling V, Purcz N, Braesen JH, et al. Comparison of two different absorbable membranes for the coverage of lateral osteotomy sites in maxillary sinus augmentation: a preliminary study. J Craniomaxillo Surg 2013;41(1):76–82. DOI: 10.1016/j.jcms.2012.10.015.
15. Mehta DB, Deshpande NC, Dandekar SA. Comparative evaluation of platelet-rich fibrin membrane and collagen membrane along with demineralised freeze-dried bone allograft in grade II furcation defects: a randomized controlled study. J Indian Soc Periodontol 2018;22(4):322–327.DOI: 10.4103?jisp.jisp_310_17.
________________________
© The Author(s). 2020 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.